Regulatory Environment and Approvals in Cell and Gene Therapy Products Between Japan, the USA, and the EU
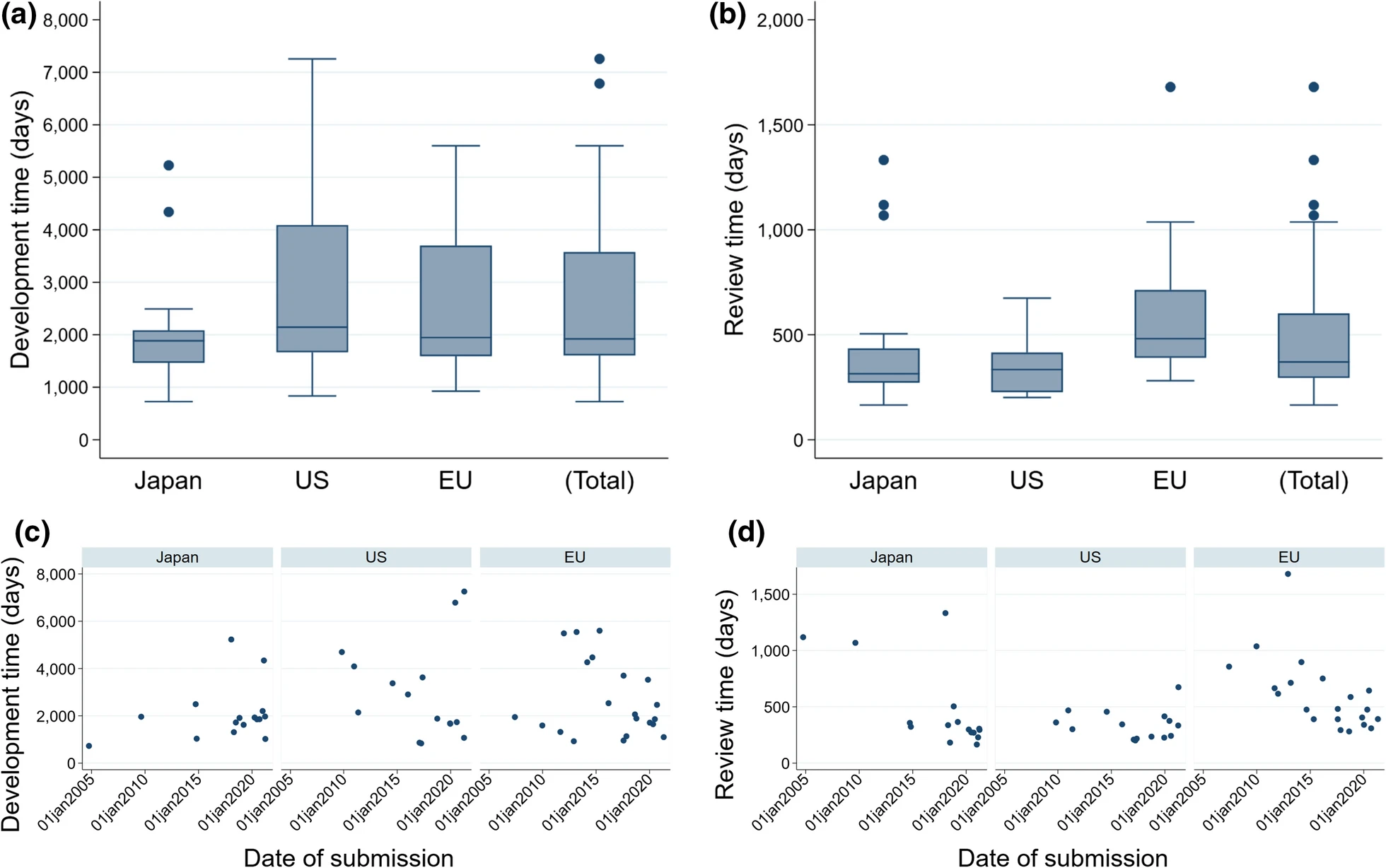
This study aimed to demonstrate the differences in the way cell and gene therapy (CGT) products have been developed and reviewed for approval in Japan, the USA, and the EU by comparing regulations and successfully launched products in each region, and to examine the background to such differences.
Submit an Abstract

Submit an abstract for an existing or future event on any topic that advances the pharmaceutical, biotechnology, medical device, and related fields.
Content Highlights
Learn More
Regulatory Affairs: The IND, NDA, and Post-Marketing On-Demand Training
Complete this On-Demand Training Course to learn about FDA regulations and expectations for the content, submission and review of INDs/NDAs, and the importance of regulatory strategy.
US Regulatory and Compliance Considerations Online Course
Increase your understanding of regulations and guidance around the dissemination of information about drug products in the US.
