Pharmacometrics: The Already-Present Future of Precision Pharmacology
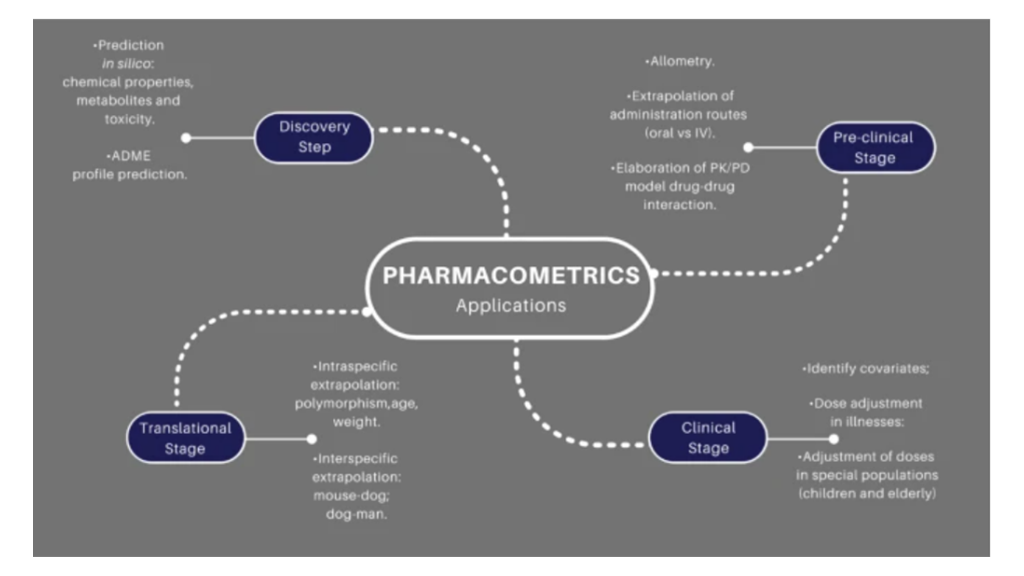
The use of mathematical modeling to represent, analyze, make predictions or providing information on data obtained in drug research and development has made pharmacometrics an area of great prominence and importance. In this review, we describe the meaning, history, and development of pharmacometrics, analyzing the challenges faced in the training of professionals.
Submit an Abstract

Submit an abstract for an existing or future event on any topic that advances the pharmaceutical, biotechnology, medical device, and related fields.
Content Highlights
-
Nonanimal Models for Preclinical Safety and Efficacy Workshops in Australia
Read Now -
Commentary: Developing Drugs for Rare Diseases: A New Approach to Generating Clinical Evidence
Read Now -
Responsible Use of AI in Healthcare: Work in Progress DIA 2024 “Responsible AI Solution Room” Summary Report
Read Now
Learn More
Discovery and Pre-Clinical Phases Online Course
In this module, learners focus on how companies advance potential drug candidates through the earliest stages of research, from therapeutic target to readiness for clinical studies.
Drug Development and Lifecycle Management eLearning Program
Understand how companies structure their efforts and utilize their resources to improve the odds of successful development, and potentially minimize the risks associated with shepherding a new drug candidate through the development process.
