Common Deficiencies Found in the Active Pharmaceutical Ingredient (API) Section of Non-sterile Generic Products Submitted for Registration by SAHPRA
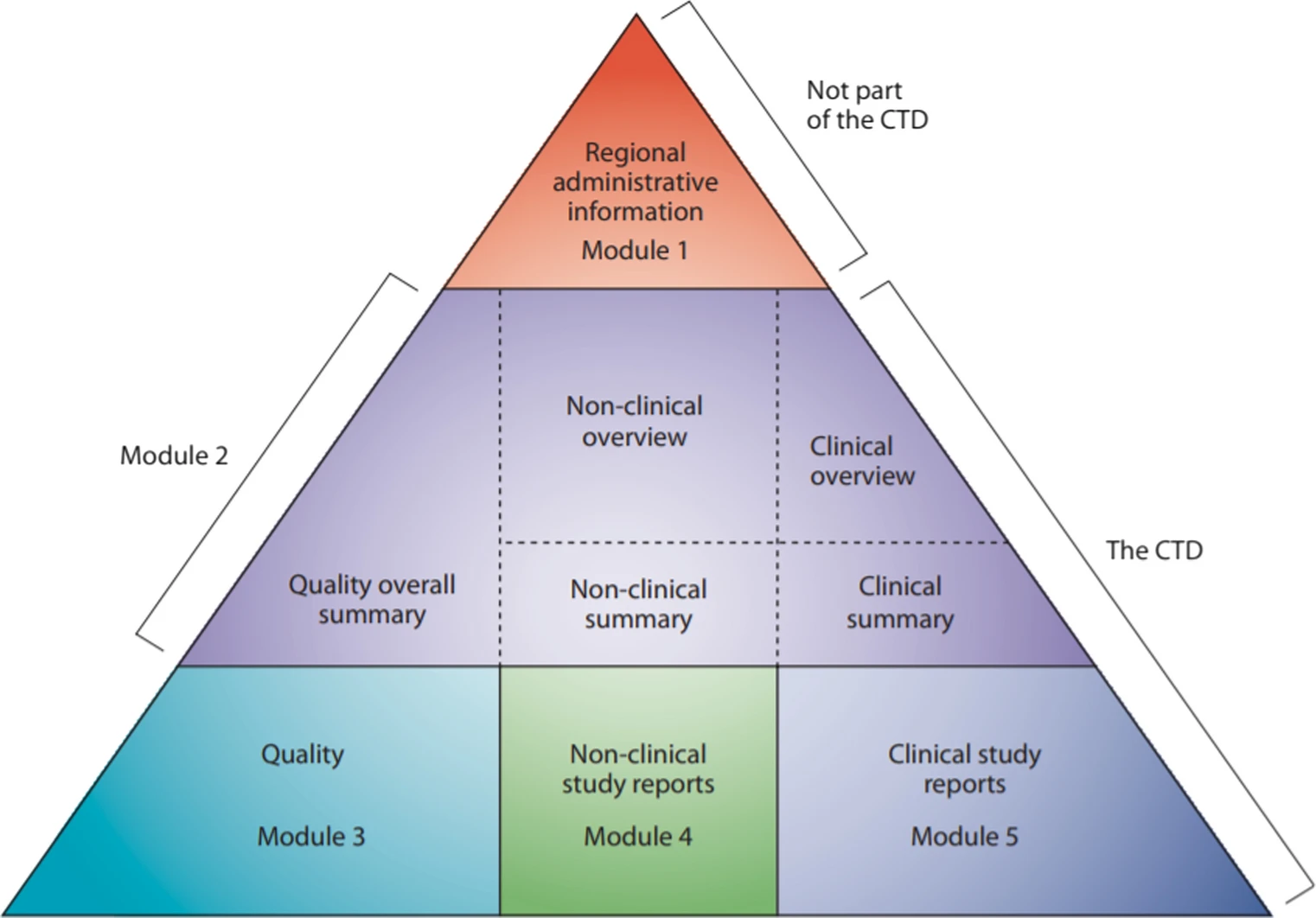
This research study aims to determine the qualitative and quantitative common deficiencies included in the API section of dossiers submitted to SAHPRA. The study was conducted retrospectively over a 7-year period (2011–2017) for non-sterile generic products that were finalised by the Pharmaceutical and Analytical pre-registration Unit.
Submit an Abstract

Submit an abstract for an existing or future event on any topic that advances the pharmaceutical, biotechnology, medical device, and related fields.
Content Highlights
Learn More
Navigating Chemistry, Manufacturing, and Controls Through the Drug Development Process Online Course
Register for this On-demand Training Course which will demonstrate CMC sections of regulatory submissions, the FDA inspection process, and how to avoid or minimize 483s (noncompliance) using real-life scenarios.
Pharmacovigilance Quality Management System Online Course
This beginner to intermediate level hands-on virtual live training course describes contemporary principles, practical approaches, and regulatory expectations for the Pharmacovigilance Quality Management System.
