Considering Global Development? Insights from Applications for FDA Breakthrough Therapy and EMA PRIME Designations
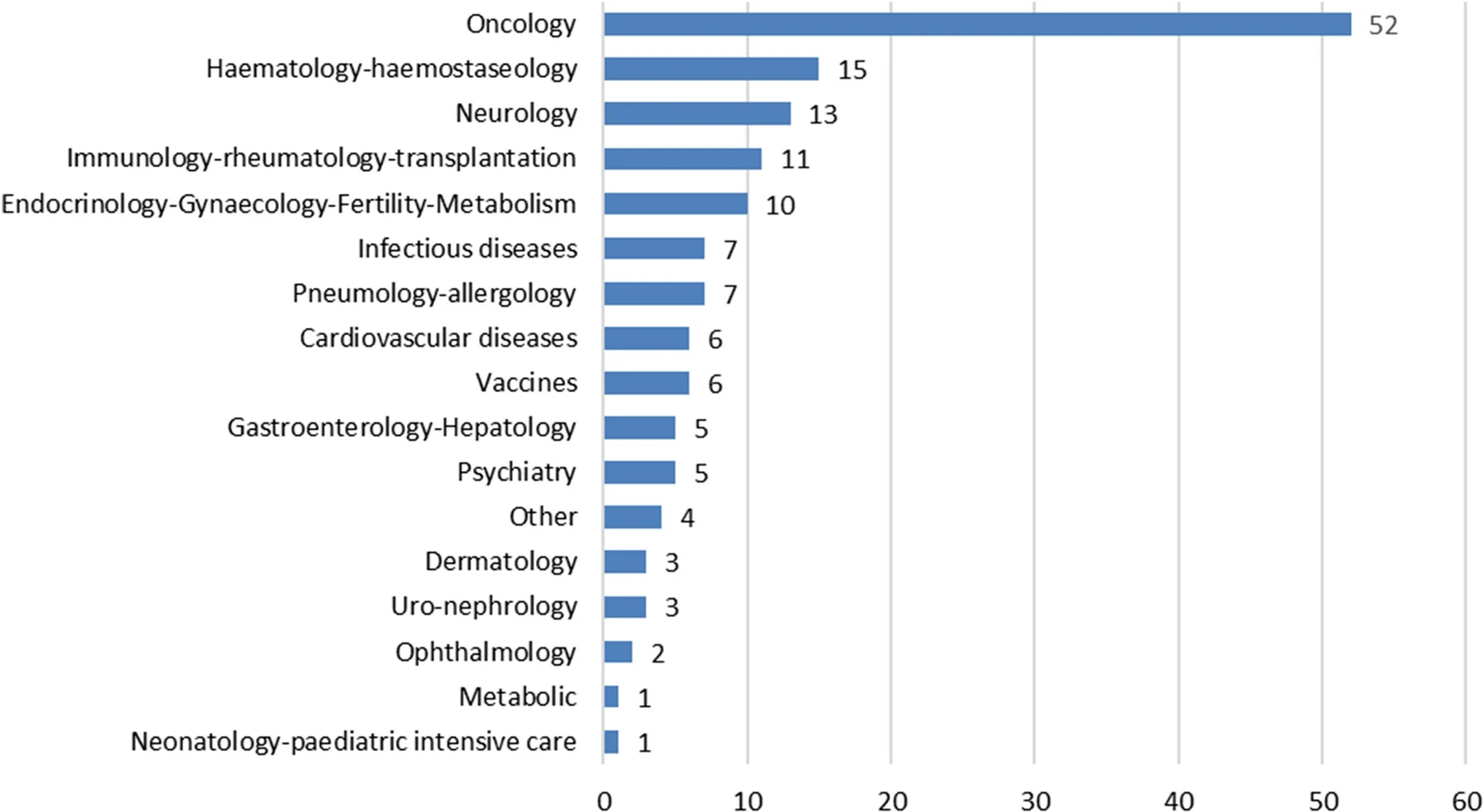
In this original research, The United States Food and Drug Administration (FDA) and the European Medicines Agency (EMA) suggest strategies for working toward better engagement in global development, and advice on taking advantage of existing collaborative opportunities, in parallel with scientific advice to identify innovative approaches that support the global development of products for unmet medical needs.
Submit an Abstract

Submit an abstract for an existing or future event on any topic that advances the pharmaceutical, biotechnology, medical device, and related fields.
Content Highlights
Learn More
Drug Development and Lifecycle Management eLearning Program
Drug development is an incredibly complex and risky endeavor, one that even experienced organizations will fail at more often than they succeed. This program will help you understand how companies structure their efforts and utilize their resources to improve the odds of successful development, and potentially minimize the risks associated with shepherding a new drug candidate through the development process. At the end of the program, you will be able to explain the phases, major work streams, key players and interrelationships necessary to develop new drugs in the US and Europe, and to expand the life cycle of in-line products.
Clinical Trial eLearning Program

The Clinical Trial Fundamentals eLearning Program is designed to provide a practical context to help clinical research professionals learn about conducting clinical trials.
