Contact Us
Learn Mored-Wise Technologies
Overview
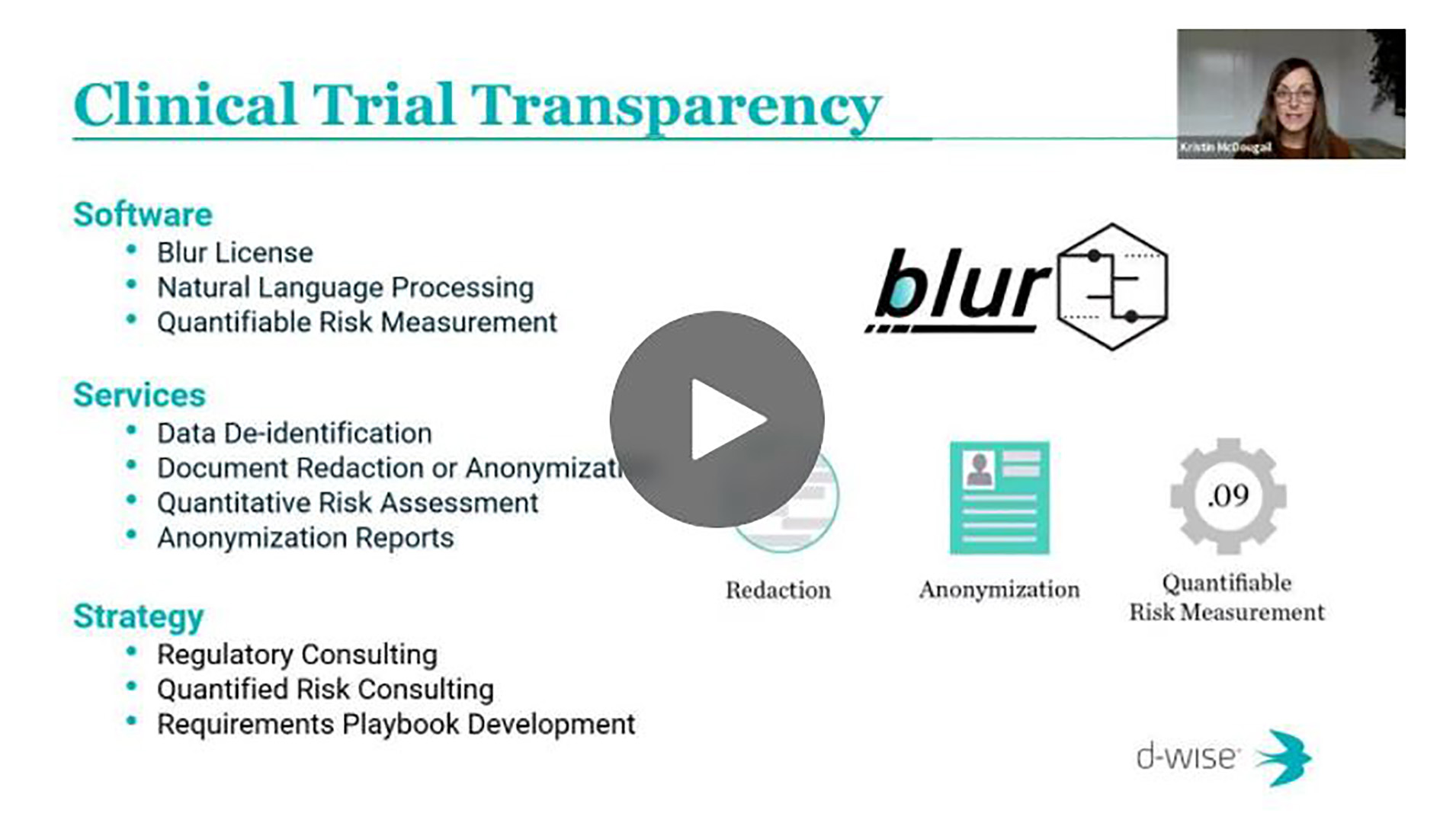
d-wise provides on-demand, flexible outsourced transparency services designed to measure risk and anonymize data and documents within strict sharing deadlines. Leveraging Blur anonymization software, our team of transformation experts provide swift data and document anonymization and quantifiable risk measurements so your organization can confidently share on any scale.
d-Wise
Blur Software

De-ID Services