Bayesian Method of Borrowing Study-Level Historical Longitudinal Control Data for Mixed-Effects Models with Repeated Measures
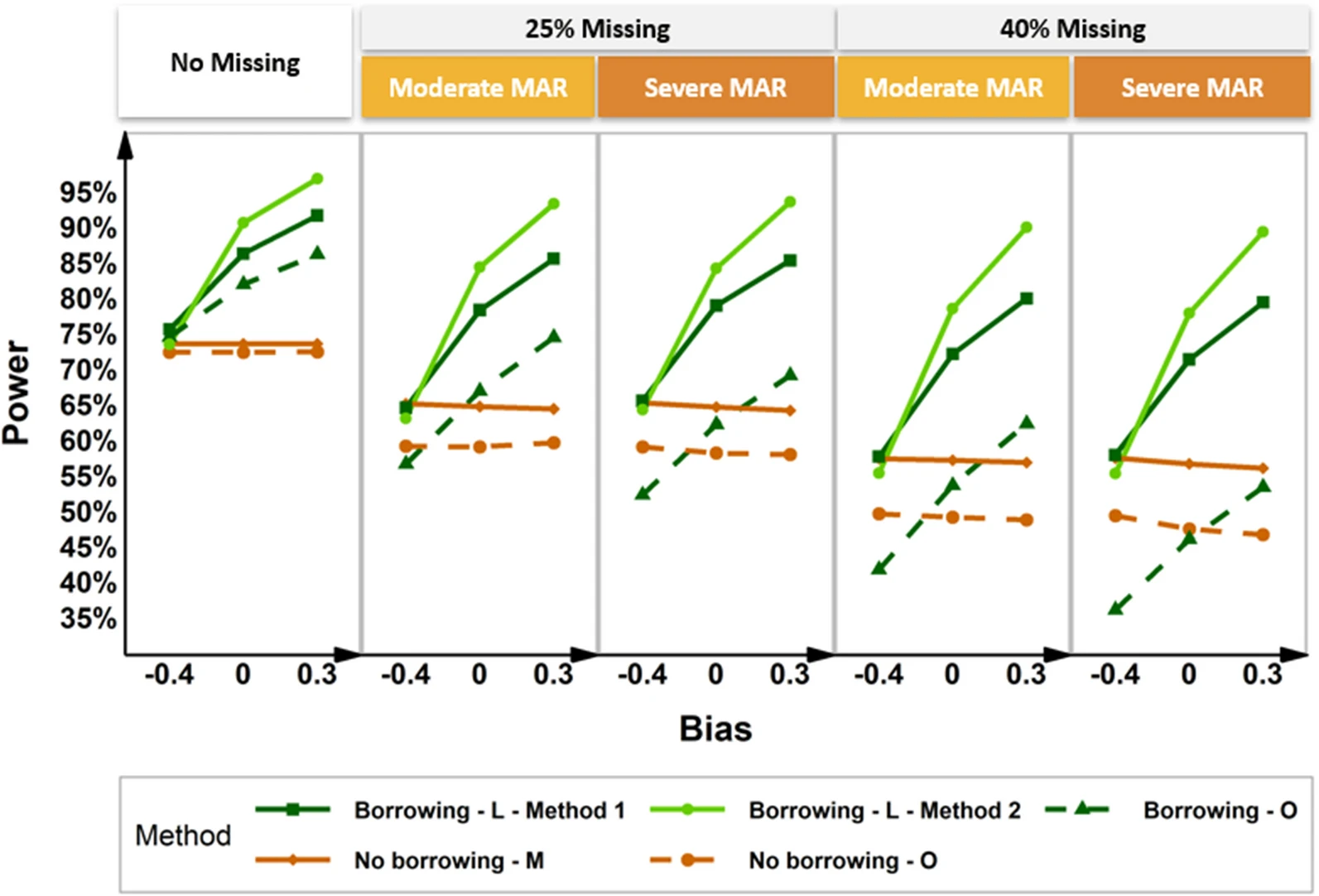
Current historical borrowing methods focus on incorporating patient-level historical control information at only one time point. In this work, we propose a Bayesian hierarchical Mixed effect Models for Repeated Measures to incorporate aggregated study-level longitudinal historical control estimates into the concurrent trial that collected repeated longitudinal data.
Submit an Abstract

Submit an abstract for an existing or future event on any topic that advances the pharmaceutical, biotechnology, medical device, and related fields.
Content Highlights
Learn More
Clinical Statistics for Nonstatisticians Online Course
Register for this On-demand Training Course to learn about confidence intervals, hypothesis testing, trial designs, and methods for establishing noninferiority and equivalence.
Statistics for Medical Affairs Online Course
Develop your understanding of how to evaluate statistical data presented in medical literature.
