News Updates
- Benefits of Integrating Patient Engagement with Diversity, Equity, and Inclusion in New Medical Product Development: A Call for Action
- Drug Shortages in the Global South: A Proposed Parallel Tech and Reg Transfer Framework
- Fair Pay for Patient Engagement: Navigating the Evolving Landscape of Remuneration
- Industry’s Window to Express Interest in Africa Continental Product Evaluation Pilot Closes End of February 2024
- The Importance of Regulatory Due Diligence During the Acquisition Process
Managing Pharmacovigilance Outsourcing Partners
What Every Biopharmaceutical Company Must Know: Choosing & Managing Pharmacovigilance Outsourcing Partners
 Biopharmaceutical companies are facing increasing pressure to develop more robust pharmacovigilance programs, with teams needing to review more sources of data, speed their response times, and track adverse events in greater detail. These forces are pushing many companies to their budgetary and resource limits, and a number of them are embracing pharmacovigilance outsourcing as one possible solution. Outsourcing gives companies access to skilled safety professionals and validated processes to handle the various aspects that their own teams are challenged to deliver, from routine case processing to specialized risk management activities, based upon the unique needs of their organization.
Biopharmaceutical companies are facing increasing pressure to develop more robust pharmacovigilance programs, with teams needing to review more sources of data, speed their response times, and track adverse events in greater detail. These forces are pushing many companies to their budgetary and resource limits, and a number of them are embracing pharmacovigilance outsourcing as one possible solution. Outsourcing gives companies access to skilled safety professionals and validated processes to handle the various aspects that their own teams are challenged to deliver, from routine case processing to specialized risk management activities, based upon the unique needs of their organization.
In 2014, 70% of global biopharma companies outsourced some part of their pharmacovigilance work, according to a report from ISR. In 2016, more than half of these companies reported outsourcing all postmarketing operations to avoid the high operational cost associated with technological infrastructure and hiring dedicated staff.
It is estimated that pharmacovigilance outsourcing, currently a two billion dollar market, will double in value within the next five years, outpacing growth in traditional outsourcing businesses such as accounting, software development, and information technology support. While outsourcing pharmacovigilance is a growing trend that can provide significant efficiencies and cost savings, such results are not guaranteed without proper planning and oversight. Regardless of who conducts the actual safety tasks, drug developers are still responsible for monitoring and reporting all associated adverse events. This means they must ensure their outsourcing partners are carefully vetted, supervised, and held accountable for results.
Tolerance and Control
An optimized outsourcing program must strike the right balance, for both internal stakeholders and service providers, between capacity, capability, and control. To achieve this balance, companies must first conduct a complete assessment of their current pharmacovigilance program, including the size and expertise of their staff, ongoing demands on their time, as well as the scope of current and pending pharmacovigilance efforts. They also need to consider which aspects of the pharmacovigilance process they absolutely must control, and their risk tolerance for activities they may be willing to outsource.In effect, biopharmaceutical leadership should conduct an analysis, such as a 2x2 matrix (figure 1), of their need for process control versus the level of risk they are willing to assume. This assessment should be undertaken for the organization as a whole, as well as at the individual stakeholder level, to identify areas of alignment and illuminate potential areas of incongruity, which can be quite disruptive once service delivery is underway. The specific results of this analysis will vary for every company based on the maturity of their products, the experience of their staff, and their “cultural” level of comfort with outsourcing arrangements.
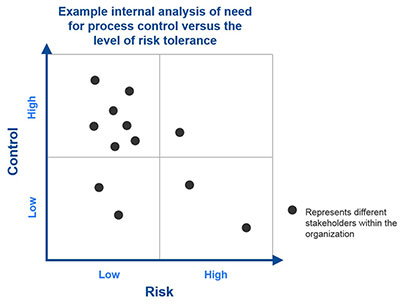
Companies with a lower risk tolerance may opt to outsource very specific tasks or subsets of tasks, such as individual case data entry for mature products only, while retaining control of their entire end-to-end process. On the other hand, companies with a higher tolerance for risk, or those with limited internal expertise, may outsource an entire process such as the authoring of an aggregate report from start to finish, thereby building strategic partnerships with their vendors, who can act as extensions of their in-house team.
This internal assessment process is the most critical step in beginning an outsourcing initiative. Fully understanding internal organizational needs and requirements will clarify not only what activities to be outsourced, but also provide insights into possible partners and outsourcing models that best meet company needs. For example, companies that want to maintain a high degree of control over their pharmacovigilance programs will likely want vendors who can follow client standard operating procedures (SOPs), use client databases, and employ client internal experts to make the more demanding decisions. Conversely, a company that is interested in maintaining less daily operational control may prefer a vendor who can act more autonomously, follow their own SOPs, utilize their own databases, and insert their own medical expertise.
Implementing an Outsourcing Model
After an organization has clearly defined its risk tolerance and which tasks to outsource, they can build an outsourcing business plan defining roles, responsibilities, oversight activities, and measures for success, to prepare them to begin vetting potential partners. These early conversations should explore what expertise both sides bring to the table, what processes will be used, how governance will be conducted, and the frequencies and format of communication and monitoring control. Companies should work with their partners to define risks related to outsourced activities and appropriate mitigation strategies. It is important to build this risk management plan as a joint team, and to revisit it regularly, to ensure that these strategies remain relevant and that both parties are meeting the overall goals of their joint program. Needs can change as partnerships mature.Ongoing governance and oversight is critically important when outsourcing pharmacovigilance. Regardless of who is conducting the actual tasks, it cannot be emphasized enough that the biopharmaceutical company will always remain accountable to regulatory authorities for the actual results achieved.
To make the most of these outsourced relationships, these companies should carefully choose a single internal point of contact to monitor and manage the overall relationship – and they should choose carefully. While many companies assume this role should be taken on by the in-house safety expert, that approach may not be the right fit, especially if that person is uncomfortable with the outsourcing model or lacks vendor management experience. Regardless of the choice, additional job training and support can help close any gaps and create a positive relationship between the vendor and in-house team.
Whether a company outsources some aspects of their pharmacovigilance process, or the entire process, taking the time to assess the needs and risk tolerances, develop clear outsourcing strategies, and build strong partnerships with vendors, is crucial. It will ultimately help companies add value for their internal stakeholders, their products, and most importantly, their patients.
References available upon request.
