Want to learn more?
If you or your organization want to learn more about DIA’s Research projects or Think Tanks please contact Science@DIAglobal.org.
Institutionalizing Innovation
Diversity, Equity, and Inclusion
Despite the recognition that appropriate diversity in clinical trials is an imperative, the development of guidance from regulatory authorities, and the emergence of several efforts that illuminate the gaps, there is still a significant dearth of practical resources to guide the implementation of best practice.
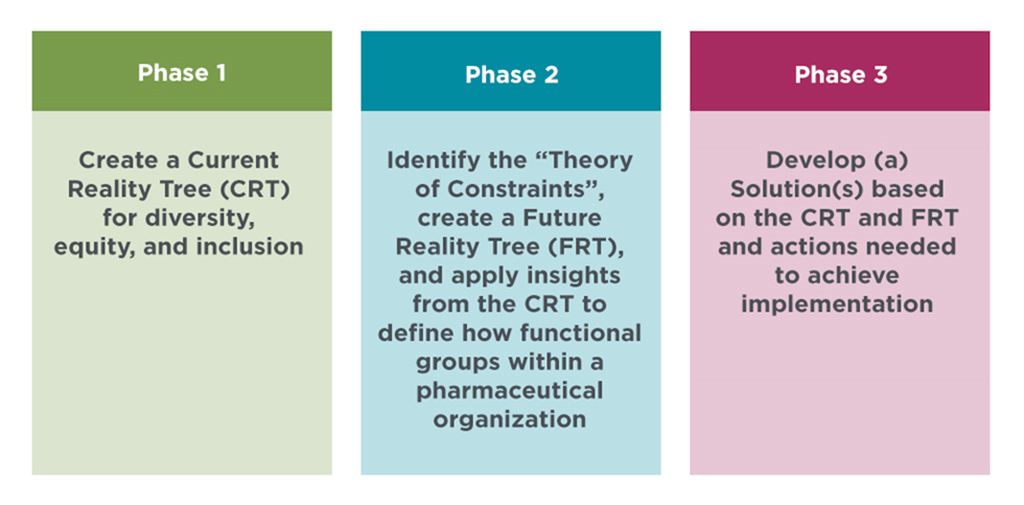
To understand the challenges, organizational barriers, desirable and undesirable effects (UDEs), DIA is convening a series of workshops under the Think Tank umbrella. The goal of the Think Tank is to create an action plan for implementation of appropriate representation and equity in clinical trials and research. The goal will be achieved in three phases:
How might our ecosystem achieve alignment on non-urgent indications that could benefit from approaches that led to success in responding to the pandemic?
The goals of this think tank are to convene a global consortium, achieve alignment on other indications that could benefit from approaches that led to success in the development of vaccines and therapies for COVID-19, to develop recommendations for an enhanced risk-based regulatory framework. Developing in collaboration with DIA members to generate:

To learn more about DIA’s Think Tanks, please contact science@diaglobal.org
Think Tank: Shortening the Focal Length to Implementing Diversity, Equity, and Inclusion in Clinical Research & Development
In the realm of life sciences research and development, progress is measured not solely by groundbreaking treatments, but also by the ability to ensure that these innovations reach every corner of our society. Picture a medical breakthrough that could save lives but is only tested on a narrow slice of the population. This is the critical issue we face in the realm of clinical trials, a lack of diversity that has far-reaching implications for healthcare and patient outcomes.
At the margins of the DIA Annual Meeting 2023, stakeholders from different regions and industry experts came together for a DEI DIAmond session and Solution Room to discuss strategies, challenges, and successful and unsuccessful stories to advance diversity in clinical trials. The key takeaways include the need for a willingness to adapt, embrace experimentation, and learn from failures. The approach should involve a broad spectrum of participants, including industry, academia, regulators, and patient communities. Self-reflection and focusing on the long-term benefits of the system are essential. Collaboration is crucial, but it should be tailored to the unique needs of each stakeholder. Lastly, achieving meaningful representation in trials requires a conscious, collective effort from everyone involved, recognizing that each drug development program may require different approaches to DEI.
To access the Outcomes report, click here.